BT-474 Cell Line Derived Xenograft
BT-474 is a human breast cancer cell line that was established from a primary tumor in a patient with breast cancer. It is commonly used as a model for studying the biology of breast cancer, which is the second most common cancer in women worldwide and is associated with a significant mortality rate. The BT474 cell line has several characteristics that make it a useful tool for cancer research. It is able to grow in culture and exhibits many of the same properties as breast cancer cells found in patients. Researchers have used the BT-474 cell line to study the molecular mechanisms of breast cancer, including the genes and pathways involved in cancer cell growth, invasion, and metastasis. The BT-474 cell line has also been used to test the efficacy of various cancer treatments, including chemotherapy, radiation therapy, and targeted therapies. It has been particularly useful in the development of drugs that target the human epidermal growth factor receptor 2 (HER2), which is often overexpressed in breast cancer.
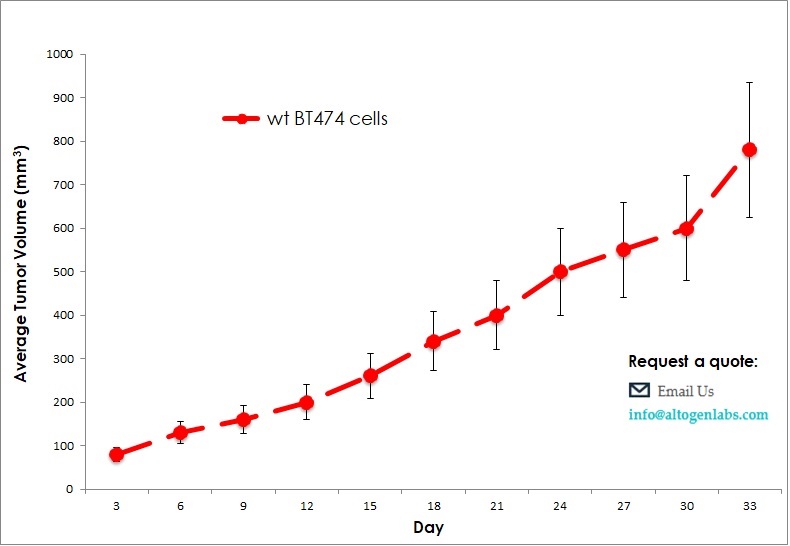
The use of the human BT-474 cell line derived xenograft (CDX) model is imperative for evaluating novel cancer therapeutics, as breast cancer is the seconding leading cause of death in women. Breast cancer in humans is extremely heterogeneous, thus, any therapeutic studies performed in vitro must be followed by a xenograft study. The combination of the appropriate immunocompromised mouse with BT-474 human cancer cells enables the researcher to study efficacy of their lead compound on tumor growth inhibition (e.g. apigenin, anti-PVRL4, HSP90 inhibitors).
| BT-474 | ER+, PR+, HER2+/-, p53(mut) |
| Origin | Breast |
| Disease | Ductal Carcinoma |
| Metastatic Models (Breast) | 4T1 |
| Non-Metastatic Models (Breast) | BT-474, HS578T, KPL-4, MCF-7, MDA-MB-157, MDA-MB-231, MDA-MB-453, MDA-MB-468, T-47D |
Get Instant Quote for
BT-474 Xenograft Model
Why use Xenograft Models?
Cell line derived xenograft (CDX) models or patient derived tumor xenograft (PDX) models enable a larger realm of parameters to be studied not capable with in vitro studies. The complete animal system model expands the scope of studies available to include the effect of test compounds on pharmacokinetics (PK), pharmacodynamics (PD), alternate routes of delivery, inhibition of metastasis, CBCs, dosing regimens, dose levels, etc. However, one of the major drawbacks of CDX and PDX models is that the human cancer cell lines or human patient derived tumors must be implanted in immunocompromised mice in order to bypass the graft versus host rejection by the animal. With the increasing focus of the immune systems role in the recognition and elimination of tumor cells (i.e. immunotherapy), major consideration must be taken into account during experimental concept design of the limitation of checkpoint inhibitors or desired immune response involvement in tumor efficacy. Similarly, any tumor regression after treatment with a test compound in these models will not exhibit the potential complement cascade or innate immune response of the injected therapeutic in humans.
What we offer?
Our in vivo xenograft service department evaluates the efficacy of preclinical and clinical cancer therapeutics utilizing more than 50 validated immunocompromised xenograft mouse models. The value of utilizing our xenograft service department is highlighted by the ability to completely characterize the efficacy, dose regimen, dose levels and optimal combination ratios of lead compounds for cancer, obesity, diabetes, infections and immunology research.
During the design and execution of the xenograft study, our scientists will communicate with and assist the client’s decisions regarding these details:
- Study Group Formation: classification of mice by body weight, tumor size or other parameters
- Cancer Cell Line: use of in-house cell lines or utilization of customer-provided cell lines
- Tumor Implantation: intraperitoneal, subcutaneous, submuscular or intravenous
- Test Compound Administration: intraperitoneal, intravenous, tail vein, subcutaneous, topical, oral gavage, osmotic pumps or subcutaneous drug pellets
- Sample Collection: Tumors/tissues can be fixed in 10% NBF, frozen in liquid N2 or stabilized in RNAlater; blood chemistry analysis can be performed throughout the in-life portion of study
Vivarium
Our vivarium is designed such that it enables cost-effective and first-rate preclinical effectiveness testing services. All animal handling and maintenance is regulated following IACUC guidelines. Our facility consists of the following:
- IACUC-regulated and GLP-compliant
- Controlled, limited access lab areas
- Disposable cages
- Sterile food and water
- SPF (specific pathogen-free) animals to guarantee pathogens do not interfere with the experiment
- Established animal handling and micro-injection equipment systems, including an animal health observation program
- All studies follow pre-approved SOPs
Our staff understands that each proposed study design is unique and customized to the client’s needs. We also recognize the importance of the delivered results as being confidential, highly reproducible and that 100% of the intellectual property (IP) is owned by the client.
In order to receive a quote for your xenograft study, email us the specific details listed below in order to efficiently begin the study quote process:
- Cancer cell line(s) used in the study
- Number (n=) of animals in each study group
- Number of study groups and control groups
- Tumor implantation route
- Administration route of test compound
- Species of immunocompromised mouse (e.g. NOD/SCID, athymic Nude)
- Treatment and dose schedule
- Study endpoint and analysis (e.g. tumor growth delay, PK/PD, survival, toxicity, drug combinations)
- Samples collected: tumor and tissues to be collected, including storage condition (e.g. snap frozen, RNAlater, 10% NBF, nucleic acid isolation)