Calu3 Cell Line Derived Xenograft
Calu-3 cells are a valuable cell line in biomedical research due to their utility in studying lung epithelial cells and for investigating drug absorption and metabolism in the lung. They are a widely used model system for investigating the molecular mechanisms of lung cell biology and for developing new drugs and therapies for lung diseases and disorders.
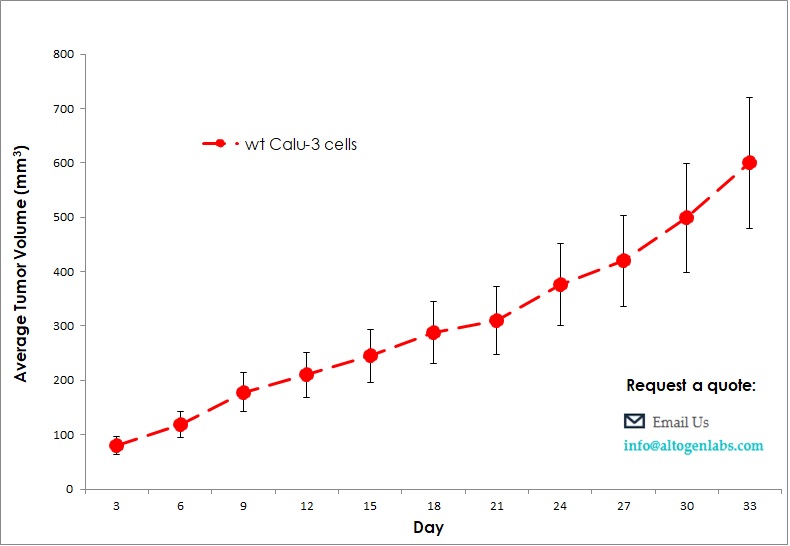
Therapeutic activity from a tumor growth delay study is utilized as a model of clinical disease. The Calu-3 CDX mouse model enables investigation of human lung cancer cell therapeutics targeting migration, apoptosis, anti-angiogenesis and anti-tumor growth in a human-similar tumor environment. Such tumor growth delay studies include the combination studies of erlotinib and cetuximab, or trastuzumab and pertuzumab. Calu-3 is a human lung adenocarcinoma cell line that is commonly used in cancer research. Xenograft refers to the transplantation of human cancer cells or tissue into immunodeficient mice or other animals for further study. Therefore, Calu-3 xenograft refers to the use of Calu-3 cells in a mouse model for studying the growth and behavior of lung cancer. This type of research is useful for testing the effectiveness of new drugs or treatments, as well as for understanding the molecular and cellular mechanisms involved in cancer growth and metastasis. In a typical Calu-3 xenograft experiment, researchers would inject the cells into the mouse and monitor the growth of the resulting tumor. They may then treat the mice with various drugs or therapies to see how the tumor responds. This type of research can provide valuable insights into the biology of lung cancer and help to identify new targets for therapy.
| 53(mut) | |
| Origin | Lung |
| Disease | Adenocarcinoma |
| Metastatic Models (Lung) | A549 |
| Non-Metastatic Models (Lung) | Calu-3, Calu-6, H1155, H460, LL/2, NCI-H1975, NCI-H226 |
Get Instant Quote for
Calu-3 Xenograft Model
Xenografting is a technique used to study human cancer and evaluate potential new treatments. This involves transplanting human tumor cells into an animal, and the model is classified according to the site of the transplant. This can be used to determine how effective a drug is and how toxic it is. Metastasis models have also been modified to predict cancer progression. Additionally, models have been developed from tumor tissue from patients, creating personalized medicine models. These xenograft models are beneficial for drug discovery, as they save time and money, and provide data that can be used to support clinical trials. The current use of these models for drug discovery is discussed.
The development of an anti-cancer therapeutic necessitates a well-planned, systematic process in order to succeed. In vitro studies are conducted to provide a high-throughput screening and assessment of various compounds of interest. This procedure allows for a specialized compound screening process of multiple cancer cell lines within a specific type of cancer or across a wide range of cancer types. Nonetheless, the results of the in vitro screening ought to be confirmed in an animal model since cells cultured on plastic in vitro are not able to replicate the microenvironment of a tumor.
The next step in therapeutic development is the administration of a test compound in a living animal. To this end, a cell line derived xenograft model (CDX) is created by introducing human cancer cell lines into test animals. When the injected cell lines form established tumors, it is possible to assess the efficacy of the test compounds. An alternative to CDX models is the patient derived tumor xenograft (PDX) model, which involves implanting human tumor fragments directly into a mouse model. This method avoids potential issues with CDX models, as the tumor is not grown on plastic and there is no selection of single cell populations. In contrast to CDX models, PDX models are designed to preserve the cell population, structure, and stroma of the original tumor.
In simpler terms, CDX and PDX models allow us to study a wider range of parameters than we could with in vitro studies, such as the effect of drugs on the body. However, these models have their drawbacks, as the human cancer cell lines or tumors must be implanted in immunocompromised mice. This means that we can’t properly study the immune system’s role in recognizing and eliminating tumor cells, or the response that the injected therapeutic would have in humans.
What we offer?
Our in vivo xenograft service department evaluates the efficacy of preclinical and clinical cancer therapeutics utilizing more than 90 validated immunocompromised xenograft mouse models. The value of utilizing our xenograft service department is highlighted by the ability to completely characterize the efficacy, dose regimen, dose levels and optimal combination ratios of lead compounds for cancer, obesity, diabetes, infections and immunology research.
During the design and execution of the xenograft study, our scientists will communicate with and assist the client’s decisions regarding these details:
- Study Group Formation: classification of mice by body weight, tumor size or other parameters
- Cancer Cell Line: use of in-house cell lines or utilization of customer-provided cell lines
- Tumor Implantation: intraperitoneal, subcutaneous, submuscular or intravenous
- Test Compound Administration: intraperitoneal, intravenous, tail vein, subcutaneous, topical, oral gavage, osmotic pumps or subcutaneous drug pellets
- Sample Collection: Tumors/tissues can be fixed in 10% NBF, frozen in liquid N2 or stabilized in RNAlater; blood chemistry analysis can be performed throughout the in-life portion of study
Vivarium
Our vivarium is designed such that it enables cost-effective and first-rate preclinical effectiveness testing services. All animal handling and maintenance is regulated following IACUC guidelines. Our facility consists of the following:
- IACUC-regulated and GLP-compliant
- Controlled, limited access lab areas
- Disposable cages
- Sterile food and water
- SPF (specific pathogen-free) animals to guarantee pathogens do not interfere with the experiment
- Established animal handling and micro-injection equipment systems, including an animal health observation program
- All studies follow pre-approved SOPs
Our staff understands that each proposed study design is unique and customized to the client’s needs. We also recognize the importance of the delivered results as being confidential, highly reproducible and that 100% of the intellectual property (IP) is owned by the client.
In order to receive a quote for your xenograft study, email us the specific details listed below in order to efficiently begin the study quote process:
- Cancer cell line(s) used in the study
- Number (n=) of animals in each study group
- Number of study groups and control groups
- Tumor implantation route
- Administration route of test compound
- Species of immunocompromised mouse (e.g. NOD/SCID, athymic Nude)
- Treatment and dose schedule
- Study endpoint and analysis (e.g. tumor growth delay, PK/PD, survival, toxicity, drug combinations)
- Samples collected: tumor and tissues to be collected, including storage condition (e.g. snap frozen, RNAlater, 10% NBF, nucleic acid isolation)