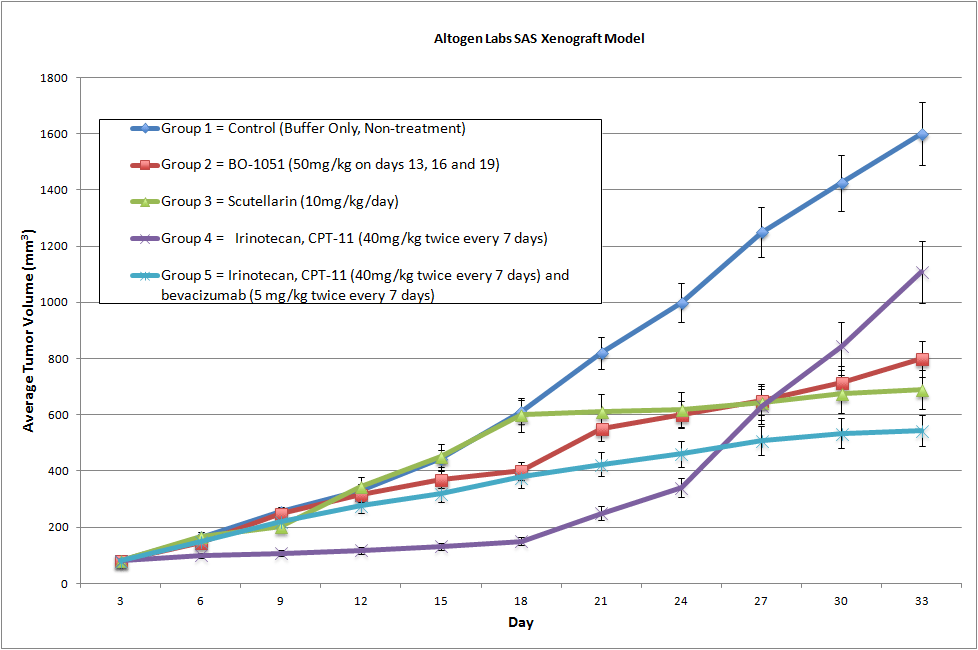
SAS Xenograft Model
SAS oral cancer, also known as squamous cell carcinoma of the oral cavity, is a type of cancer that affects the mouth and throat. It typically starts in the flat, thin cells that line the inside of the mouth and can spread to other parts of the body if left untreated. SAS cells are known to exhibit many of the characteristics of oral squamous cell carcinoma, including abnormal cell growth and migration, resistance to apoptosis (programmed cell death), and the ability to invade surrounding tissue. They are often used as a model system to study the mechanisms underlying oral cancer progression and to test potential therapeutic agents for their ability to inhibit cancer growth and metastasis. The most common risk factors for SAS oral cancer include tobacco use (including smoking and smokeless tobacco), heavy alcohol consumption, and infection with the human papillomavirus (HPV).
Over 50 validated xenograft animal models are available to conduct contract research based drug development studies for oncology, inflammation, diabetes, infectious diseases, obesity, immunology and pain research. Xenograft services are used to assess the effectiveness of drugs against various types of cancer in various stages. These drugs are tested on staged tumor growths that have been engrafted subcutaneously or orthotopically in immunocompromised mouse or rat models. There isn’t a clinically approved anti-cancer agent that hasn’t been tested positively in conventional preclinical in vivo models.
Our standard xenograft models are generated via following process:
- Administration of tumorigenic cell lines via subcutaneous or orthotopic injection
- Administration of therapeutic agents according to predetermined schedule
- Monitoring animal health and tumor growth
- Performing necessary testing, including blood analysis, pharm/tox, imaging, biodistribution, PK/PD
- Performing gross necropsy and tumor processing (biochemical and IHC analysis)
Our state-of-the-art testing facility offers cost-effective laboratory services. Our facilities are:
- GLP-compliant
- IACUC-regulated
- Barrier facility with disposable cages, autoclaved food and water
- An SPF (specific pathogen-free) installation
- Established quality system that includes health observation program
If you would like to receive xenograft study quote, please e-mail us key project details, including a) the cell line(s) to be used for the study, b) number of animals for each group, c) number of treatment and control groups, d) test compound administration route, e) type of immunocompromised species (usually NOD/SCID or athymic Nude mice), g) treatment schedule, f) endpoint and type of analysis (tumor growth delay, biomarker expression analysis, PK/PD, LD50, etc).
Get Instant Quote for
SAS Xenograft Model
SAS Basic Tumor Xenograft Study Design
NOD/SCID or Nude (Nu/Nu) mice (8-12 weeks old) are acclimated to the facility for 5-7 days prior to tumor cell injection/implantation.
Cells to be used for injection are maintained under conditions of exponential growth prior to injection. Cells are prepared for injection by trypsinization and viable cell counts are determined using trypan blue exclusion.
Cell count performed by flow cytometry and suspension adjusted to appropriate density. Only cell suspensions with greater than 98% viability are used.
Each mouse receive one subcutaneous injection in the flank of the hind leg.
Each site will receive one million tumorigenic cells in a volume of 100 uL containing 50% matrigel (use of matrigel matrix improves tumor cell survival and tumor formation).
The injection sites are palpated three times weekly until tumors are established. Tumors are measured using digital calipers until they reach an average size of 150-250 mm3. Injected animals are monitored daily with cage-side observations, and body weight measurements.
Animals are randomized into treatment cohorts and treated according to the treatment schedule. We recommend including cyclophosphamide or other positive control treatment group.
Once palpable tumors are observed, tumors are measured and mouse weights recorded 2 times a week.
Animals are euthanized when tumor size reaches 2,000 mm3 size limit. Gross necropsy and tissue collection performed as defined for termination of experiment. Animals showing signs of morbidity due to tumor burden or treatment toxicity are euthanized and processed as defined for termination of experiment.
Tumors are excised, weighed and documented by digital imaging. Standard necropsies are performed and tissues collected for downstream processing and analysis.